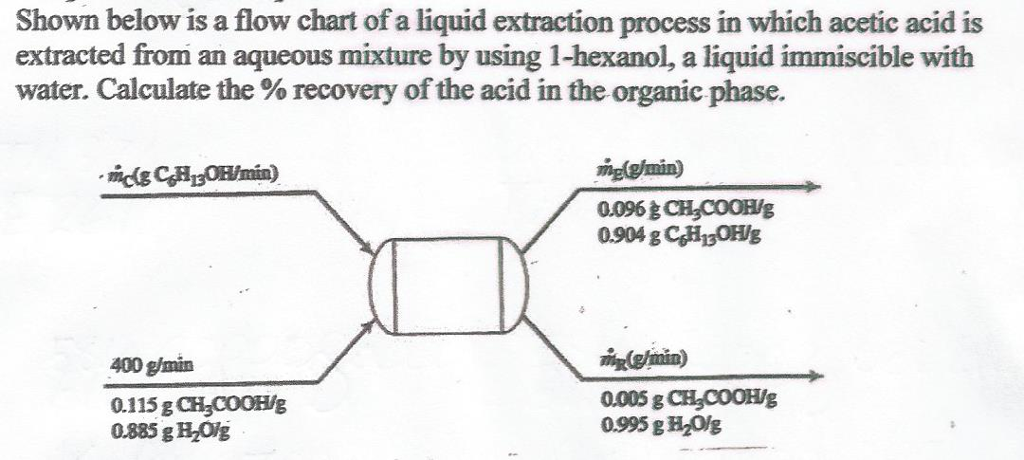
Liquid Liquid Extraction Process Flow Diagram
Liquid liquid extraction lle also known as solvent extraction and partitioning is a method to separate compounds or metal complexes based on their relative solubilities in two different immiscible liquids usually water polar and an organic solvent non polar.
Liquid liquid extraction process flow diagram. Cannabis extraction cannabis extraction process flow. If the components of the original solution distribute differently between the two liquids separation will result. Cannabis harvest dehydration packing storage shredding supercritical co2 extraction molecular distillation solvent solvent recovery crystallization desolvation cbd crystals heating reactor liquid cbd tags. There is a net transfer of one or more species from one liquid into another liquid phase generally from aqueous to organic.
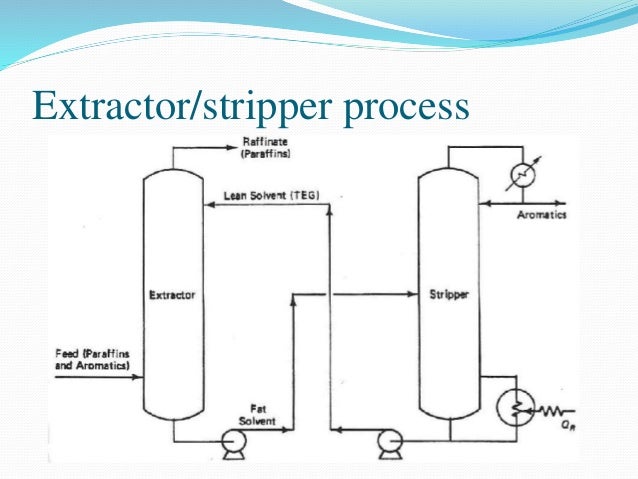
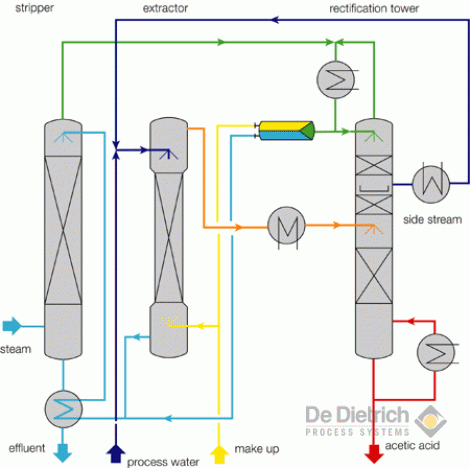
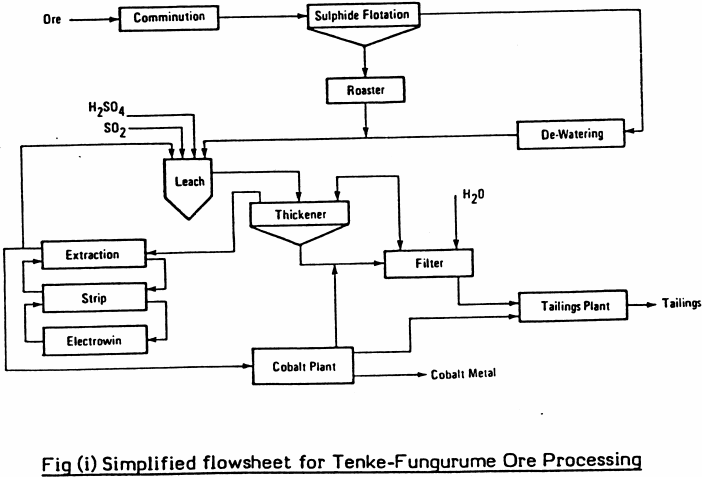
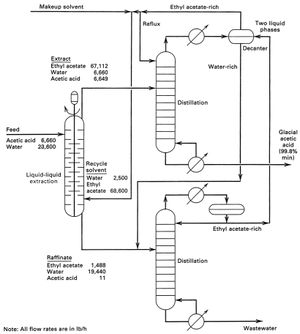
One of the key decisions when designing an extraction process is the choice of the solvent to be used. Liquid extraction produces separation of the constituents of a liquid solution by contact with another insoluble liquid. A schematic diagram of a complete liquid liquid extraction process from separation process engineering by wankat 2007 in the extraction process the feed which contains the first solvent or the diluent and the solute is sent to the extraction unit or the extractor another solvent commonly called the solvent. In this liquid liquid phase triangular diagram the mixtures to be.
L 02 1 1 v l vm 1 a. L 00 2 2 1 1 1 1xv a y a l xv a y a mx am 2 c. L 0 0 2 2 11 11xv cc c c cm y l xvy mx 3 since x a x b x c 1 an equation for b is not needed. Because l 0 and v 2.
Process flow diagram cannabis extraction. It was located in the organic layer during the extraction process. The following flow chart shows the general procedure for isolating the three compounds benzoic acid 2 naphthol and. A process flow diagram b phase diagram.
Extract layer and raffinate layer. Liquid liquid extraction lle also known as solvent extraction and partitioning is a method to separate compounds or metal complexes based on their relative solubilities in two different immiscible liquids usually water polar and an organic solvent non polar. Courses learning chemical engineering.